Measurement systems
To measure the quantity of anything, we need a comparison with some precise unit value. Early humans used body parts and natural surroundings to provide suitable measuring instruments.

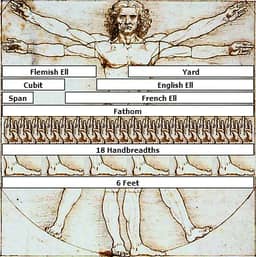
Historical units of measurement
This derivation of the Vitruvian Man by Leonardo Da Vinci shows nine historical units of measurement. Da Vinci drew the Vitruvian Man to scale, so the units shown here are in their proper historical ratios.
Rudimentary measures became essential in primitive human societies for tasks such as building dwellings, making clothing, bartering for food and exchanging raw materials:
Early Babylonian and Egyptian records show that length was first measured with the forearm (cubit), hand (palm and span) and the finger (digit).
The cycles of the Sun, moon and other celestial bodies were used for time measures.
Plant seeds were used to establish volume measure, and with the development of scales for weighing, seeds and stones served as standards. For example, the carob seed was the base measure for the carat, which is still used as a mass unit in the gemstone industry.
As trade and commerce expanded, it became necessary to standardise measurement systems, not only within a given country but also between countries. This reduced the likelihood of disagreements arising from measurement system misunderstandings.
Nature of science
Empirical observations made in the course of scientific investigations often involve measurements of some quantity. It is only through a system of standard, agreed units of measure that scientists worldwide can effectively communicate their findings.
The English system
A Weights and Measures Act (1824) redefined common units of measurement in England. Before this, standardisation was only achieved through royal decree:
Edward II (1324) defined the inch as 3 barleycorns.
Henry I (1130) decreed that 1 yard is the distance from the tip of his nose to the end of his outstretched thumb
Queen Elizabeth I (1575) altered the mile to 5280 feet so it became 8 furlongs (1 furlong = 220 yards).

Public standards of length
This plaque shows British imperial standards of length. The plaque was mounted outside the Royal Greenwich Observatory in 1866. The public used the standards to check the accuracy of their rulers.
The 1824 Act standardised the system of units used across the British Empire. It is known as the imperial system based on the foot-pound-second.
The French system
During the French Revolution (1789–1799), the French Academy of Sciences was asked to “deduce an invariable standard for all the measures and all the weights”.
The Academy decided on two founding principles. The system would be:
based on scientific observation
a decimal or base 10 system.
In this system:
the unit of length – metre = one 10 millionth of the distance from the North Pole to the equator along the meridian running near Dunkirk in France and Barcelona in Spain
the unit of mass – gram = the mass of 1 cm3 of water at its temperature of maximum density. 1 L of water has a mass of 1 kg at this temperature.
The new calendar consisted of 12 months of 30 days each, with a 5–6 day holiday to complete the 365-day year. Each day was divided into 10 hours, 100 minutes per hour and 100 seconds per minute, but the new calendar was never actually used.
The National Assembly adopted the metric system, based on the metre-kilogram-second, on 7 April 1795. It only became compulsory throughout France in 1840.
Other countries recognised the superiority of the metric system. In 1875, 17 countries signed the Treaty of the Metre. This treaty established the setting up of three separate but linked organisational structures:
The General Conference on Weights and Measures (CGPM) – a formal body responsible for ratifying any new proposals on metric units.
The International Committee for Weights and Measures (CIPM) to oversee scientific decisions.
The International Bureau of Weights and Measures (BIPM) to oversee the activities of the national standards laboratories. The headquarters of BIMP were set up in Sèvres, France.

Badge of BIPM
The badge of the International Bureau of Weights and Measures (BIPM) represents an allegory of science holding the new metre standard with its decimal divisions. The badge carries the inscription in Greek ‘metro kro’ or ‘use the measure’.
By 1900, 35 countries had officially adopted the metric system.
The SI system
In 1960, representatives of nations using the metric system attended the 11th General Conference on Weights and Measures. An extensive revision and simplification of the system was agreed to, and six units were established as base units for the system, called Le Système International d’Unités (International System of Units), abbreviated to SI. These units were the metre, kilogram, second, kelvin, ampere and lumen.
In 1971, another base unit was added – the mole as base unit for the amount of substance.
Standard units like the metre, kilogram and second make it possible to trade and communicate throughout the world without misunderstanding.
An international community of metrologists (people who study measurement) is constantly improving the SI measurement system. For example, on 16 November 2018, the 26th General Conference on Weights and Measures agreed to redefine the kilogram, the ampere, the kelvin and the mole. The new definitions are based on fundamental constants: Planck's constant, the Avogadro constant, the Boltzmann constant and the charge of the electron. On 20 May 2019, the changes came into effect and the wording of the other three units was updated.
Measurement – a timeline
- Observational practices
- Technological advances
- Developing international standards
- 3500 BC
Harappan mass units
Gary Todd, CC0 1.0
The Harappan Civilization, living in the Indus River Valley (north-western regions of modern-day South Asia), create fire-baked bricks that are uniform in size and shape. The bricks are used to build baths and sewerage. Bricks with these same dimensions appear in multiple cities across the region. Collections of balance weights in the form of stone cubes have been found and dated to 2800–2600 BC. The smallest of these weights measured 0.87 g, but the most commonly found example weighed 13.65 g, which suggests that this was the basic mass unit for the Harappan.
Image: Harappan (Indus Valley) balances and weights.
- 3000 BC
Sumerian numeral system
The ancient Sumerians, living in what we now call southern Iraq, use a numeral system with 60 as its base. It is believed to have been derived from their astronomical observations. The Sumerians’ sexagesimal system is still used in measuring angles, geographic coordinates, electronic navigation and time.
- 2750 BC
The cubit
Public domain
The cubit, considered as the first recorded standard length measurement, appears in ancient Egypt. It is defined by the length of the Pharaoh’s forearm, as measured from the tip of his forefinger to the middle of his elbow. There are multiple examples throughout history of length measurements based on various body parts. One ‘hand’ is measured across the widest part of the palm including a closed thumb. It is still used to measure the height of horses. Today, one hand = 4 inches = 101.6 mm.
Image: Fragment of a cubit measuring rod.
- 2100 BC
Royal gur-cube
The Sumerians have a royal gur-cube, which is a theoretical cuboid of water, measuring approximately 6 × 6 × 0.5 m. They use this to derive their other measurement units.
- 1600 BC
Water clocks
Egyptians and Babylonians use water clocks (clepsydra) to measure the passage of time. Some authors claim that water clocks were used in China as early as 4000 BC.
- 1500 BC
Sundials
Public domain
Egyptians use sundials to measure time by tracking the movement of the sun via the length and position of shadows cast on a marked circular surface. It is very likely that humans used this time-tracking method from a much earlier date in our history, but exact details are difficult to verify.
Image: Ancient Egyptian sundial from Egypt’s Valley of Kings.
- 800 BC
The foot measurement
The ancient Greeks and Romans use the foot to measure length. In Greece, its size can range between 270 mm and 350 mm, depending on the location. The standard Roman foot is normally about 295.7 mm, but in the provinces, a longer length of about 334 mm is used. Today’s foot is somewhat longer than the original Roman foot and is now equivalent to 304.8 mm or 12 inches.
- 220 BC
Circumference of the Earth
Eratosthenes, a Greek mathematician and scientist, attempts to determine the circumference of the Earth. He is told that, at midday on midsummer, the Sun shines straight down a particular well in Aswan, a city in the south of Egypt. At exactly the same time in Alexandria (a city in Egypt’s far north), he observes that the Sun casts a shadow 7.2° from the vertical. By timing the journey by camel between the two cities and knowing the average distance covered in a day’s walk, Eratosthenes calculates that the Earth must be 46,000 km around. Today, we know that the value is closer to 40,000 km, so he is out by just 15%.
- 70 BC
Predicting astronomical events
Joyofmuseums, CC BY-SA 4.0
Ancient Greeks build the Antikythera mechanism – an early version of an analogue computer. Its purpose is to predict astronomical positions and eclipses decades in advance.
Image: Antikythera Mechanism, National Archaeological Museum, Athens.
- 100
Land survey tool
Greek inventor Hero writes about a system called the dioptra, meaning the spyhole. It is a mechanical system that measures distances and angles between objects. It is used as a land survey tool by the Romans when planning large-scale projects like roads and aqueducts. The dioptra is very similar to a modern theodolite.
- 700
Hourglass
A French monk makes an hourglass to measure time. They become commonplace in the 14th century.
- 1400
Localised measurement standards
Richard Mortel, CC BY 2.0
Highly localised measurement standards are in common use. In European towns and cities, specific statues act as length standards for traders. The Dubrovnik ell measures 51.2 cm and is defined as the length of the forearm on a statue of Orlando (a mythical knight). The Bremen ell in Germany is measured between the knees of a similar statue, but its measure is 55.9 cm.
Image: Orlando Column, Dubrovnik.
- 1500
Māori measurement
Measurement standards, most often based on the human body, are used to construct wharenui, waka and woven articles with a high degree of precision. Time is measured with the phases of the Moon – 30 nights of the Moon are identified and named.
Image: Wharenui.
- 1750
The Industrial Revolution
The Industrial Revolution transforms all aspects of daily life, turning rural societies into those dominated by large-scale industry and urbanisation. Large deposits of coal and iron ore drive these developments, providing an alternative source of energy to traditional human power. The first practical steam engine is developed in 1713, and by the turn of the century, improved versions of the engine power machinery, trains and ships. These innovations in transportation, paired with the invention of the telegraph, make the world much smaller and speed up communications. Globalisation begins, and with it comes the need for reliable, accurate measurement.
- 1791
Unified measures
In the immediate aftermath of the French Revolution, the French Academy of Sciences, which includes several pre-eminent scientists, is commissioned to create unified and rational measures based on a decimal system. The Marquis de Condorcet, the permanent secretary of the Academy, says that this new system should be “À tous les temps, à tous les peuples” (For all time, for all people). The unit of length, the metre, is defined as 1/10,000,000th the length of the quadrant of the Earth’s meridian passing through Paris (i.e. the distance from the equator to the North Pole), and a survey is undertaken to determine this measurement. This leads to a definition of the unit of mass – the kilogram, which is the mass of a cube of water with dimensions 0.1 × 0.1 × 0.1 m (i.e. 1 litre) of pure water at 4°C.
- 1795
The metric system
Public domain
The metric system is formally written into French law. It defines six new decimal units, only two of which (the metre and the kilogram) are retained for later metric systems.
Image: Woodcut illustrating the new decimal units.
- 1799
Physical standards of mass and length
The survey to determine the distance from the equator to the North Pole is completed. Physical standards of mass and length are made using platinum and deposited in the French National Archives.
- 1812
Redefining old units
Despite a law requiring everyone in France to use the new system, older systems remain popular. Napoleon revokes this law and issues one called the ‘mesures usuelles’, which restores many of the old units but redefines them in terms of the metric system.
- 1832
Measuring time
German mathematician and physicist Carl Friedrich Gauss makes absolute measurements of the Earth’s magnetic field in terms of the millimetre, the gram and the second. Gauss’s second is defined in terms of observations of the Earth’s rotation, which can be described in terms of the ancient Sumerians’ sexagesimal (base 60) system. This is where we get the 60-second minute, 60-minute hour and 24-hour day from.
- 1863
Base units and derived units
Public domain
British scientists, including James Clerk Maxwell and William Thomson (later Lord Kelvin), suggest a common measurement system is needed – one based on the metric system using base units and derived units. Maxwell proposes three base units: length, mass and time.
Image: Lord Kelvin.
- 1874
Use of prefixes
The British Association for the Advancement of Science proposes the centimetre-gram-second (CGS) system and begins to use prefixes like micro- and mega- to describe very small or very large quantities.
- 1875
Metre Convention
Badge of BIPM, reproduced with permission of the BIPM, which retains full internationally protected copyright. © BIPM.
Representatives of 17 nations gather together in Paris to sign the Metre Convention (or Metric Treaty) – an agreement to use the metric system for the kilogram and the metre. It also creates the BIPM, the International Bureau of Weights and Measures, which is now considered the global home of measurement. Shortly afterwards, 30 new prototypes of metre and 40 prototypes of kilogram are cast using a platinum-iridium (Pt-Ir) alloy. 20 May is now referred to as World Metrology Day.
Image: Badge of BIPM, reproduced with permission of the BIPM, which retains full internationally protected copyright.
- 1876
Trafalgar Square examples
Kaishu Tai, CC BY-SA 3.0
Brass plaques are installed in London’s Trafalgar Square showing the imperial units of the foot, 2 feet and 3 feet (yard).
Image: Imperial standards of length in Trafalgar Square.
- 1881
Electrical units of measurement
The first International Conference of Electricians adopts the British Association for the Advancement of Science definition of the ohm and adds definitions for the volt and the ampere, amongst other electrical units.
- 1889
International prototypes deposited in Paris
Greg L, CC BY-SA 3.0
The international prototype metre and international prototype kilogram are selected at random from the 70 artefacts cast in 1875. These two are deposited into a vault in the basement of the BIPM in Paris. The remaining prototypes are distributed amongst the member states. These new standards are sanctioned.
Image: International prototype kilogram.
- 1921
Metre Convention extended
The scope of the Metre Convention is extended to include all aspects of the metric system.
- 1948
The candela
The University of Waikato Te Whare Wānanga o Waikato
A new ‘standard candle’ (luminous intensity) – the candela – is defined. The measurement describes the intensity of artificial light sources as they appear to the human eye. The name makes reference to an original standard – the light produced by a standard candle.
- 1952
The astronomical second
The astronomical second is adopted as the standard for time.
- 1954
The kelvin
The kelvin is established as a measurement of thermodynamic temperature. It is based on the triple point of water – an unchanging property of water at which water, ice and water vapour co-exist in equilibrium.
- 1960
The ampere
The University of Waikato Te Whare Wānanga o Waikato
The ampere – measurement of electric current – is redefined. The new definition is related to the force per unit length between two very long parallel wires. The definition resembles the original experiment carried out by André-Marie Ampère, the scientist for whom the unit is named.
1960SI units are formally adopted
The name SI (International System of Units) is formally adopted. It initially includes six physical base units – metre, kilogram, second, ampere, kelvin and candela.
- 1971
The mole is added
The mole (amount of substance) is added to the SI, creating the current system of seven units. One mole is initially defined as 12 grams of pure carbon-12.
- 1991
Zetta and yotta
New metric prefixes – zetta and yotta – are added to the International System of Units (SI) to enable chemists to express vast molecular quantities.
- 2015
MSL’s Kibble balance
Measurement Standards Laboratory of New Zealand, a business of Callaghan Innovation.
The New Zealand Measurement Standards Laboratory (MSL) begins work to develop a Kibble balance that is much simpler than existing Kibble balances. The Kibble balance is an electromechanical instrument that measures mass. MSL has the only Kibble balance in the southern hemisphere.
Image: Dr Sutton with the kibble balance.
- 2019
Redefining the kilogram, the ampere, the kelvin and the mole
Reproduced with permission of the BIPM, which retains full internationally protected copyright. © BIPM.
On World Metrology Day (20 May), widespread changes to the SI come into effect. Four of the base units – the kilogram, ampere, kelvin and mole – are redefined based on fixed values for some fundamental constants of nature, including Planck’s constant. The wording of the remaining three units is also updated.
Image: The redefined SI (International System of Units).
- 2022
New metric prefixes
In November 2022 international scientists voted for new metric prefixes to express the world's largest and smallest measurements, prompted by an ever-growing amount of data.
Welcome to ronna and quetta for the largest numbers – and ronto and quecto for the smallest.
Measurement – a timeline
This timeline explores measurement – its development from local units to a standard international system (SI). A full transcript is underneath the timeline.